You are here :
- Unité de recherche
- LIRA
- Home
- Discover LIRA-CY
who are we?
Laboratoire d'Instrumentation et de Recherche en Astrophysique
LIRA-CY
The understanding of a large number of astronomical observations (from the Earth's upper atmosphere to the most distant galactic objects) and their astrophysical interpretation depend on basic atomic and molecular data. These data result from studies and laboratory experiments carried out under conditions simulating, while simplifying them, the physico-chemical conditions of the environments observed. It is in this triple aspect of scientific knowledge (observation - experimentation - interpretation) that the LIRA-CY Laboratory at the CY Cergy Paris University is interested in, in collaboration with the Paris Observatory within the general framework of the joint research unit 8254- LIRA (Astrophysics Instrumentation and Research Laboratory).
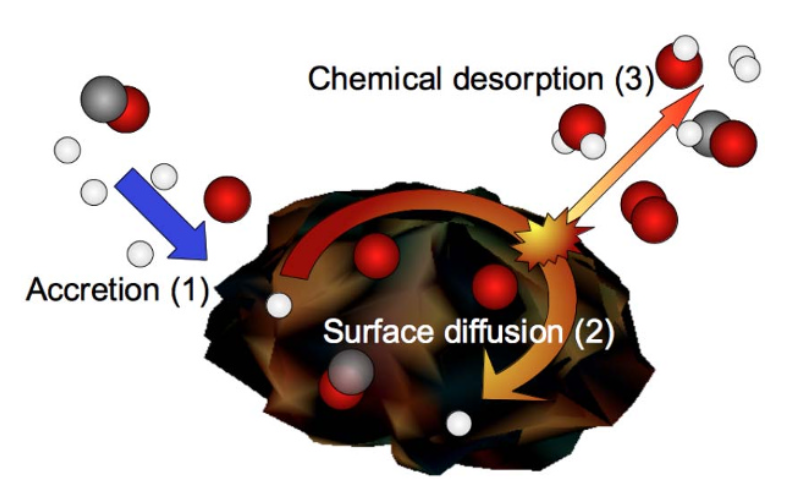
Context: How do molecules form on cold surfaces?
The molecules of our daily life, such as water or carbon dioxide, existed long before the birth of the Earth. Radio-astronomical observations are able to trace this chemical prehistory, especially if the molecules are in the gas phase. But it is in the solid phase that complex molecules are probably synthesized, and are therefore very difficult to observe. This is why laboratory astrophysics must explore the synthesis of molecules on cold surfaces, and thus provide the necessary answers concerning this fundamental aspect hidden from new observations. To this end, we are building specific experimental devices dedicated to this theme.
The Reactivity on Cold Surfaces team is an experimental physics team hosted by the University of Cergy Pontoise, which is interested in the evolution of atoms and molecules on surfaces of astrophysical interest. It studies in particular the reactivity of atoms and molecules but also all the processes associated with it, such as bonding, diffusion and desorption.
The team uses atomic and molecular jets that they interact with surfaces (graphite, silicates, ice, etc.) that can be cooled to very low temperatures (> 6 K) in order to place themselves in the extreme conditions of the interstellar medium.
Experimental devices
The team has two complementary instrumental devices to conduct its studies.
- UHV.
- Surfaces: 3 movable samples (e.g. graphite, gold, silicate). Surface temperature control: 10-800K.
- 2 atomic or molecular beams aimed at the sample (e.g. H, N, O, CO,…).
- In-situ controlled water ice growing system (amorphous, porous, crystalline…) or carbonaceous dust deposition (e.g. PAH coronene).
- Mass spectrometry (its use is fourfold) : Beam compositions, During Exposure Detection, Thermally Programmed Desorption, Internal energy of atoms or molecules.
- Reflection Absorption Infra Red Spectroscopy.
- Laser system (REMPI 2+1) coupled with a time of flight detection.
- Angular distribution measurements.
- New sources of atoms and radicals.
VENUS – Developed since 2011
- Up to 5 atomic or molecular beams. Only 2 presently running.
- Surfaces : Rotatable sample holder with 3 surfaces.
- Temperature Range : 10-300K
- Mass spectrometry (its use is fourfold) : Beam compositions, During Exposure Detection, Thermally Programmed Desorption, Internal energy of atoms or molecules.
- Reflection Absorption Infra Red Spectroscopy.





